Tricine, electrophoresis grade, =99%
Key Features and Details
Tricine for use in electrophoresis buffers
SYNONYMS: N-Tris-[hydroxymethyl]methylglycine; N-[2-Hydroxy-1,1-bis(hydroxymethyl) ethyl] glycine
CAS: #
5704-04-1
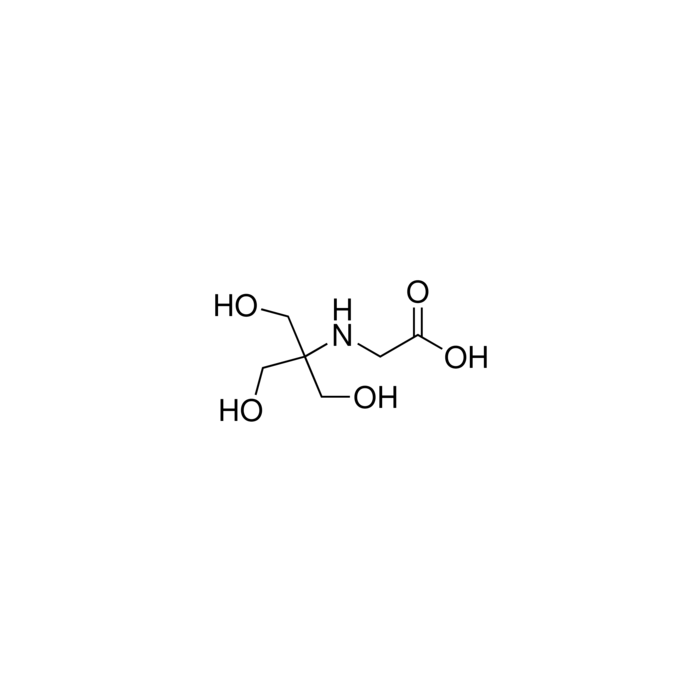
MOLECULAR FORMULA: C6H13NO5
BEILSTEIN REGISTRY No.: 1937804
EC No: 227-193-6
You need to register an account with us
to be able to place an order, Click to Register.
PRODUCT DESCRIPTION
Tricine
Application Notes
Tricine is used as buffer component for separation of low molecular weight peptides. Tricine can be used in cryopreservation medium for the preservation of tissues and organs. Cryopreservation depends on the physical and chemical characteristics of the preservation medium used. The pH values and pK values for tricine/DMSO mixtures has been reported down to -20 °C. Tricine has been found to be an efficient scavenger of hydroxyl radicals in a study of radiation-induced membrane damage. Tricine is typically the buffer of choice in SDS-PAGE systems when separating proteins in the range of 1 to 100 kDa.
Usage Statement
Unless specified otherwise, MP Biomedical's products are for research or further manufacturing use only, not for direct human use. For more information, please contact our customer service department.
SPECIFICATIONS
SKU: 04807410-CF
Base Catalog Number: 807410
Alternate Names: N-Tris-[hydroxymethyl]methylglycine; N-[2-Hydroxy-1,1-bis(hydroxymethyl) ethyl] glycine
Beilstein Registration No
: 1937804
CAS
: #
5704-04-1
EC No
: 227-193-6
Format
: Powder
Grade
: Electrophoresis
Hazard Statements
: H317
Insoluble Matter
: =10 ppm
Molecular Formula
: C6H13NO5
Optical Rotation: DpK/DT: -0.021(Lit.); Metal Binding Constants (log K) for 0.1 M solution at 20 °C: Mg2+: 1.2 Ca2+: 2.4 Mn2+: 2.7 Cu2+: 7.3(Lit.)
pKa
: 4.5 - 5.5 (1% aq soln) ; Buffering pH range 7.4-8.8(Lit.)
Potency
: 8.1 (25 °C)(Lit.)
Purity
: =99%
Sample Source
: Source
Very soluble in water (25% w/v - clear; colorless solution).
Storage
: 24
Unit Definition
: A buffer may be prepared by titrating with sodium hydroxide to the desired pH, using about a half-equivalent of NaOH.